By: Carlos Navarro
What’s up! My name is Carlos Navarro and I am a rising 5th year Ph.D. student studying chemistry at USC. I am a member of Travis Williams’ lab where I work on developing an effective recycling method for carbon fiber reinforced polymer composites, or CFRPs for short.
CFRPs are a mouthful to say and sound uncommon, but there’s a really good chance you’ve interacted with one recently! We are using them to build all kinds of products and goods, from airplanes to bicycles, because they have a higher strength-to-weight ratio than steel. This is great news for airplanes because we can improve fuel efficiency by using CFRPs to make them lighter, and its great news for consumer goods like golf clubs and bicycles because they are sleek, perform great, and last a long time.
This, however, is bad news for the environment, because there is no effective recycling method for CFRPs when we’re done using them! The best methods being developed right now involve shredding the CFRP into chips and heating them in giant furnaces at 700 °C, which recovers only a fraction of its value for re-use. We need to do better, which is why I’ve been working on this project for the past 5 years!
“Why are CFRPs so difficult to recycle?”, you may be asking yourself. Firstly, great question! Second: it all comes down to the polymer which holds the composite together. CFRPs are manufactured by layering sheets of carbon fiber weaves and smothering the stack in an epoxy polymer – just like the ones sold at hardware stores as a pack of two bottles. These polymers are extremely durable and non-reactive, which is great when you’re in an airplane made from CFRPs, but not-so-great when you want to recycle the airplane afterwards. This is a problem that we’re going to be facing in the near future!
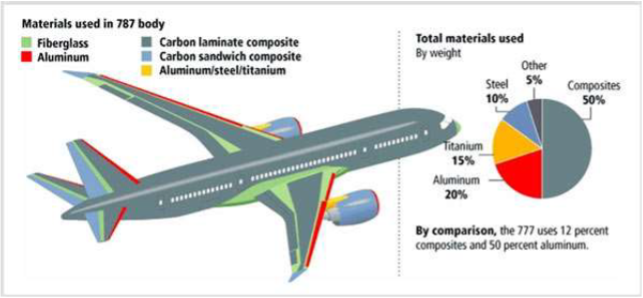
Newer generation aircraft like the Boeing 787 are 50% by weight CFRPs! Thankfully airplanes have about 30 year service lives, but we will need to deal with this waste eventually…
Since the root of the CFRP recycling problem is chemistry, we’ve decided to use chemistry to try to solve it! My research focuses on finding mild reaction conditions to undo the chemical bonds which form the polymer. Our best set of recycling conditions so far relies on using oxygen gas and metal catalysts to break the polymer bonds that are formed when it is cured, but it requires a lot of pressure. This makes the recycling reaction difficult to scale up if we want to try to make it commercially viable.
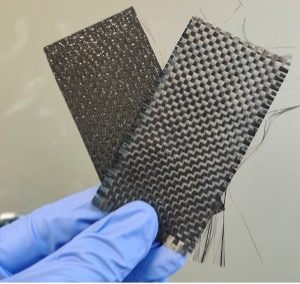
A side-by-side photo of a cured CFRP sample (left), and a recycled carbon fiber weave (right) recovered from these composites using our process. Those fiber weaves are incredibly valuable – so valuable that I have started a business for reselling them! Fingers crossed it goes well!!!
That’s why I spent the summer studying ways to either replace oxygen gas or find a way to reduce the amount of pressure. One particularly exciting project we did this summer involved making 1 cm3 polymer cubes and injecting them with a special compound which reacts with oxygen gas as it moves into the cube. We could track, in real-time, how quickly a gas was moving through a solid!! This is valuable information as I try to optimize and make this reaction as efficient and effective as possible (I wish I could share more details and photos on that, but I’ll update this blog post once we have published the paper and I’ll break it all down!).

A blue LED is connected by a conductive epoxy to a nut on the top of the reactor. We can connect the leads to an external power supply and provide light to the inside of the vessel!
This summer, I also got to use a special photo-reactor to try to recycle composites photochemically – meaning that light is also a reagent and helps destroy the polymer! With this reactor, I can illuminate the sealed interior and apply a gas pressure so I can test how much I can reduce the applied pressure while still recycling the CFRP.
I want to give a big thank you to the Wrigley Institute for selecting me for the Sonosky Sustainability Fellowship this summer – I can’t wait to make you all proud!!




