By: Lily Zhang
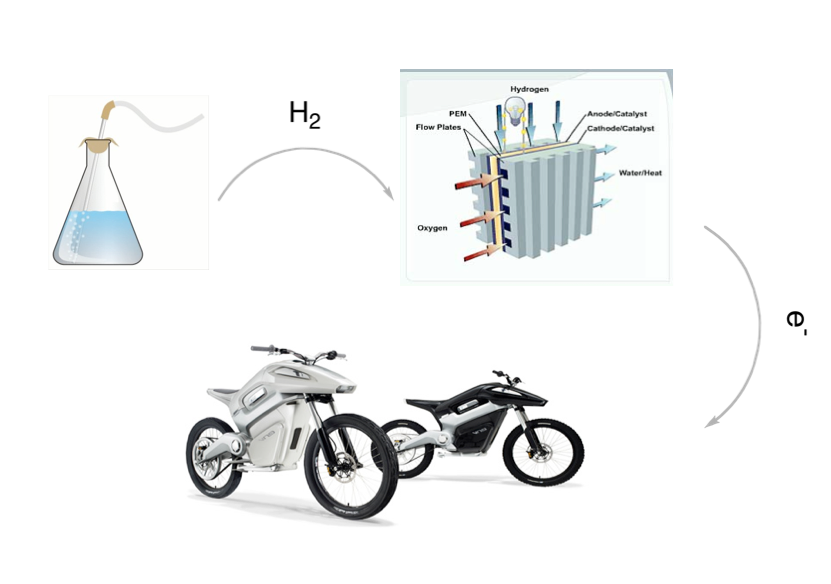
With gas prices ever on the rise and the fast consumption of our fossil fuels, we must look into transitioning from petroleum-based fuels to “greener” alternatives such as hydrogen gas (H2). Unfortunately, the current use of hydrogen as a fuel in transportation has dangers with storing the gas in a compressed state. Therefore, in my graduate research as a Sonosky Summer Fellow, I investigate alternative ways of storing the gas safely until time to release it in a controlled way.
One of the attractive options is Ammonia Borane (AB), a stable solid that is a form of chemical hydrogen storage. AB is very dense and can potentially release high amounts of hydrogen under mild catalytic conditions. Ideally, this technology would be implemented into a fuel cell where it can power light vehicles.
Our lab, the Williams Group in USC’s Department of Chemistry and the Loker Hydrocarbon Institute, has demonstrated the use of this technology using a toy car. By engineering the toy car with our catalyst systems and a fuel cell that converts H2 to fuel, we can power the car.
However, one of the problems with AB is that after a certain point, a detrimental byproduct called borazine forms. Borazine is harmful to the reaction in two ways: first, it often deactivates the catalyst, and second, its volatile nature means it can travel into fuel cells and poison them.
This summer, I searched for the optimal catalytic conditions to achieve the most efficient use of AB as a hydrogen fuel. We’ve developed a new catalyst that can release high amounts of hydrogen and minimize borazine byproduct formation. We have shown that the catalyst can also convert borazine into less volatile and harmful byproducts, all while considering cost efficiency.
Current continuing efforts in our lab are testing the reusability of this catalyst, and the exact mechanism in which it works. With a bit more additional experimentation, we hope to write a manuscript detailing this research project to share it with the research community and ultimately see this contribute to “greener” fuel applications in the world.
Lily Zhang is a graduate student with Dr. Travis Williams in the USC Department of Chemistry, and was recently a 2015 Norma and Jerol Sonosky Summer Fellow with the Wrigley Institute.