By: Cherie Ho
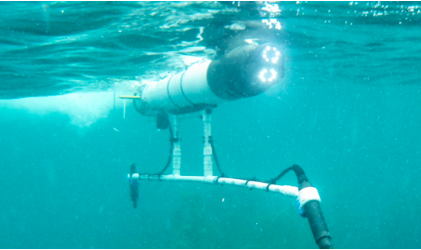
Hey! I’m Cherie and I’m a WIES Summer Fellow from Harvey Mudd College. I’m a part of LAIR, Lab for Autonomous and Intelligent Robotics. Over the years, our lab has been working with CSULB’s Shark Lab to study how we can use robots to track sharks! We were able to use multiple Autonomous Underwater Vehicles (AUVs) to track a single shark. My project this summer is to investigate how to use AUVs to track fish populations. I spent the last two weeks in Catalina deploying our robots on missions and maintaining them. Here’s the Iver, one of the underwater robot that we use for shark tracking!
To track sharks, the Iver is equipped with hydrophones that listen to acoustic pings the tags transmit. Once the Iver hears a ping, it calculates the range and bearing of the tag to estimate where the tag is. Then, it moves toward the estimated position and follows the tagged shark.
From this summer, I’ve learned that preparation is key to a successful robot deployment. Although the actual mission may take less than an hour, before that, we need to:
-assemble the Iver
-check that it is waterproof (all port holes plugged in)
-make sure the Iver’s propeller and fins are working
-calibrate sensors
-plan where the Iver can go (we don’t want to crash into kayaks and boats)
Many times, even though everything is checked and ready in the lab, the robot or the sensor may not work when we are about to deploy it at the dock. Therefore, we keep multimeters, power cables, soldering iron, glue gun and other electronics dockside to help us debug and fix the issues! We end up soldering wires a lot of times at the dock.
When everything is ready, the code is uploaded onto the Iver and we can deploy it! During the mission, we usually have someone keep track of where the Iver is. Under Catalina’s bright sun, trying to find the Iver in the water is like playing a game of Where’s Waldo.
We configure the Iver to return to its origin when the mission is over. However, sometimes the Iver may have stopped working (e.x. propeller broke) and is stuck out in the water. In that case, we use kayaks or boats to rescue the Iver.
Once the Iver is rescued, we get the data off the Iver to process later.By the time everything’s done, it’s time for food!
My summer as a Wrigley Fellow has been a rewarding experience. The Wrigley Institute is an ideal location to conduct our research due to the number and variety of marine animals available. Being able to work in the WIES makes it easy for us to deploy robots after making code or hardware changes to collect immediate results. I have learned so much more about what is involved in field robotics and got a chance to work with some amazing people. Thanks for everything, WIES! ‘Til next time.
Cherie is a graduate student from Harvey Mudd, working under Professor Chris Clark. Her summer research focused on ‘characterizing fish population movement using a model based on artificial potential fields’.