By: Bingran Cheng
Microbes are charismatic, and no, they don’t get you sick! Well, at least about 99% of them don’t. As a matter of fact, we owe our lives to these tiny single-celled organisms. I’m not just talking about the significant rise of oxygen in atmosphere about 2.3 billion years ago, thanks to the early Cyanobacteria, but also in current times. All forms of microbial cells continue to shape our environment, making the Earth a habitable place by driving the biogeochemical cycles, processes in which elements change forms from inorganic to organic and back, catalyzed by biology.
I am fascinated by the important roles that microbes play in these processes and very am interested in understanding microbial communities in their natural habitats: what species live there, how they interact with each other and how they interact with their surrounding environments, etc.
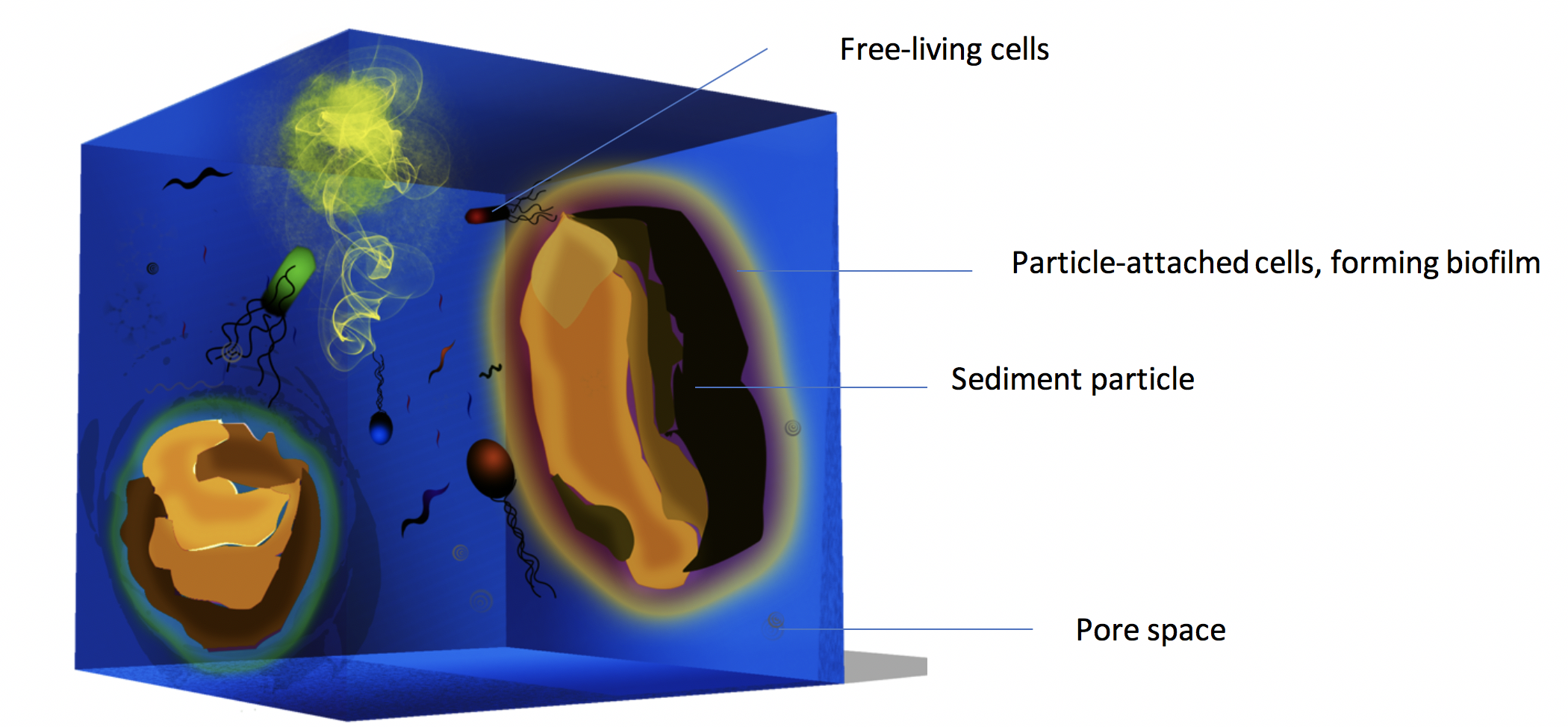
For my PhD thesis project in Dr. Wiebke Ziebis’s lab at USC, I have been studying a previously overlooked habitat — the interstitial space between the particles in marine sediments. This space, though seems trivial, takes up about 50 – 90% of total sediment volume. However, only a small portion of the benthic microorganisms live in this space, quite outnumbered by their particle-attached neighbors by orders of magnitude. Now let’s try to conceptualize the sediment frame: majority of the cells tightly attach to the sediment particles in multiple layers, forming biofilm, and there are “a few” (at least 106 cells ml-1, average cell abundance in the seawater) hanging out in the pore water. These are two completely different lifestyles, but conventional studies on marine sediments have never separated them. My goal is to bring the pore-water microbes to the center stage and take a closer look at this previously overlooked community.
I have spent quite some time at the Wrigley Marine Science Center on Catalina Island throughout the years, and this is my third summer supported by the Wrigley Summer Fellowship. Doing research at Wrigley has been an amazing opportunity for me, thanks to the help from staff and the accessibility from the lab to my sampling site, the intertidal mudflat in Catalina Harbor, I am able to collect samples, transport them back and set up experiments smoothly so that the living organisms I care about are not disturbed too much. Since I can easily walk/drive to my sampling site, I can re-visit it whenever new samples are needed. It is through these trips, I have learned quite a few exciting facts about the pore-water microbes.
My previous studies found out that the pore-water microbial communities were quite abundant, averaging 107 cell ml-1. Not only were the pore-water communities 1 to 2 orders of magnitude higher in abundance than the seawater communities, they were also 10 times more diverse than the latter. The abundant microbial groups in the seawater were rarely found in the pore water, suggesting that even though the pore water is essentially seawater, there is little exchange of microbial communities between the two compartments. The more surprising contrast was between the free-living and particle-attached microbial communities: the former rivaled the latter in diversity, with less than a hundredth abundance of the latter. The two compartments shared about half of the microbial species in the surface layer, but became more and more different with increasing depth.
The most exciting finding was that the pore-water communities were metabolically active, much more so than the particle-attached communities. Even though the pore-water communities were much lower in abundance, they were able to turn over acetate, a measure to evaluate their ability to turn over organic carbon, much faster than their particle-attached counterparts. This means that these free-living communities might play a very important role in benthic biogeochemical cycles. The wonder continues! When microorganisms oxidize organic carbon, they pass on the electrons from organic carbon to an electron donor (equivalent to us breathing oxygen), through which they gain energy for maintenance and growth. In a follow-up study to identify the electron acceptors used by the pore-water microbes, I found out that sulfate, a commonly used electron acceptor was disregarded by the free-living communities completely. The question is, why not, and what are the alternatives? With this question in mind, I went back to Catalina.
Here we begin, another summer with day-to-day adventure!
A typical working day on the island starts with sampling. I drive through a winding road with a breath-taking view to get to Catalina Harbor. I roll up my sleeves and hammer a custom-made polycarbonate tube (~ 10 cm in diameter) into the sediment, seal the top, apply a vacuum and retrieve the tube with the sediments inside (we call this a “core”), carefully keeping it undisturbed. I take the core back to lab, and the do various measurements on the core to analyze the geochemical conditions the microbes live under. One of the measurements is oxygen concentration.
I then slice the core into different depth sections. From each section, I extract the pore water using a pore-water press, which gently expel the pore water from the sediment section under nitrogen atmosphere. The pore water and the remaining pore water-free sediment are used for further experimental setup.
Today, I will try to enrich for microorganisms that “breathe” manganese oxide.
As I mentioned earlier, one of my goals this summer is to figure out alternative electron acceptors the pore-water microorganisms use for energy gain. One hypothesis is that they use manganese oxide to dump electrons they get from organic material. To test for this hypothesis, I will use a fairly straightforward method: give them manganese oxide as the sole electron acceptor, together with other essential nutrients, wait and see whether anything grows. With the help from Casey Barr (a former Wrigley Summer Fellow and a fellow USC graduate student), I have prepared some culturing medium, synthesized manganese oxide minerals in a form that is known to be liked by manganese-reducing microbes, and mixed the two together in Hungate tubes anaerobically. What I need to do today is to inoculate the pore water and the pore water-free sediment into the culture tubes and cross my fingers!
Since I am really writing this post a few days later after the day of experiment, I have news and it’s good!

Left: A set of culture tubes with enrichment medium and manganese oxide (the black particles); Right: 10 days after the day of inoculation.
Above is a picture of the culture tubes, and another taken 10 days after. See anything different? The black particles at the top of the culture tubes are disappearing in all three tubes from the left! The tube on the far right is a control, where the microbes were killed using mercury chloride so that they aren’t active at all. Therefore, the manganese oxides are really ridden by biology. So far, we can’t know for sure whether these microbes are directly reducing manganese, or if they produced something such as hydrogen sulfide that reduces manganese oxide indirectly. But it is promising.
The next steps will involve the following: start a new batch of culture with sulfate-free medium to rule out the possibility of sulfide production; use electron microscopy to verify microbial growth on the manganese particles; and identify the potential manganese reducers through 16S tag sequencing. If the pore-water microbes indeed use manganese oxide as electron acceptor, not only do they gain more energy from manganese reduction, they have also avoided direct competition with the particle-attached microbes. This niche-partition between the free-living and particle attached communities is really an ingenious example of environmental selection and adaptation.
I will be making more trips to my favorite sampling location throughout the summer and the fall. No complaints!