By: Abby Lunstrum
Hi everyone! I’m Abby – a Wrigley Fellow and first-year PhD student in the Earth Sciences Department at University of Southern California. This summer, I’ve been studying the chemistry of sandy beaches in California, and specifically how ocean acidification will alter that chemistry. Before you jump to any conclusions… let me state for the record that my work involves way more than beach towels and sun tan lotion! Keep reading to see what beach research in southern California is really like!

My advisor, Dr. Will Berelson, and I collecting sand from Cat Harbor, three miles from the Wrigley Institute on Catalina Island.
To explain what I’m researching, and why, let’s start with the big picture. Imagine earth, with several large continents surrounded by ocean. Earth is mainly a water planet, with 71% of the surface covered in water. Now imagine humans, burning fossil fuels over the past few centuries, and the product of that burning–carbon dioxide (CO2)–entering the atmosphere. We know that some of this CO2 is accumulating in the atmosphere, amplifying the greenhouse effect, and causing the warmer temperatures we’ve all been feeling the past few years. But some of it also dissolves into the ocean. Gases dissolve in water – it’s just a fact of chemistry.
So more CO2 in the atmosphere means more CO2 dissolved in the ocean. If you’ve ever used a Sodastream or other carbonator, you know this phenomenon: by putting high pressure CO2 in contact with water, you can make seltzer, which is essentially high-CO2 water. We’re doing something similar to the ocean: “carbonating” it by increasing the pressure of CO2 in the atmosphere.
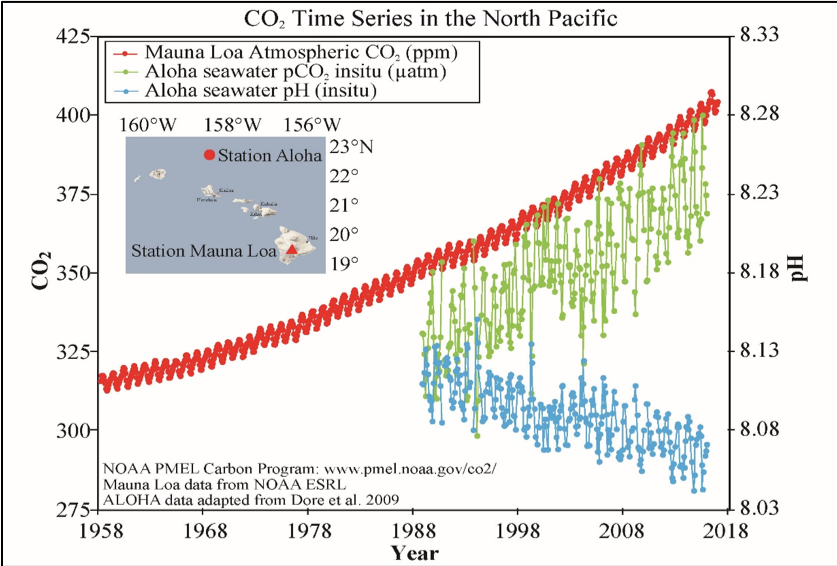
Ocean data collected at Station Aloha in Hawaii shows increasing dissolved CO2 (green line), and decreasing pH (blue line; lower pH means higher acidity) over the past 30 years. These ocean CO2 and pH trends are caused by increasing atmospheric CO2 (red line).
Scientists estimate that about a third of all the CO2 emitted from burning fossil fuels is now dissolved in the ocean. That’s good news for the climate—the earth would be even hotter now if the ocean weren’t doing us a solid—but it’s bad news for the ocean. When water absorbs CO2, it becomes slightly more acidic. And indeed, the ocean has been getting more acidic ever since we thought to start measuring it thirty years ago.
This process is called “ocean acidification.” What does ocean acidification mean for the health of the oceans? Just like you’d think, acidic water is generally not good for marine organisms, and a lot of research has shown its negative effects. For example, corals are getting stressed, oysters and clams are finding it harder to build their shells, and fish are losing their sense of smell. Those are all important things to study, but as a geochemist, I’m more interested in the fundamental chemistry of acidification, especially how acidic water might affect rocks (and vice versa, how rocks might affect acidification).
I know what you’re thinking… Rocks?! Who cares!? But hear me out…they’re important. When acidic water interacts with certain kinds of rocks (like carbonates, which I study), it dissolves them, and the result of that dissolution sequesters CO2. So dissolving rocks with acidic water could ultimately help reverse climate change! This process is a kind of feedback mechanism that the earth naturally uses to regulate the amount of CO2 in the atmosphere. But unfortunately for us, it happens very, very slowly. My research is measuring exactly how fast this occurs in beach sands (sand, after all, is essentially just tiny rocks), and how much dissolution we can expect as the ocean becomes more acidic.
To answer these questions, I’m starting local. I’ve spent the summer studying beach sands in southern California, focusing mostly on the beach in front of the Wrigley Institute. Water along the California coast is naturally slightly acidic, so it’s more vulnerable to increasing acidity, and thus a great place to study ocean acidification. To answer my questions, I’m using both lab-based and field experiments. In the lab experiments, I pump acidic water through columns of sand to replicate future ocean-beach conditions. I then measure the amount of rock (specifically, carbonate) dissolution that takes place under different acidity levels. This way, I can quantify how much dissolution—and associated CO2 sequestration—will take place over the next 25, 50, or 100 years. The field experiment uses a similar approach: I insert chambers into the sand and acidify the overlying water. As the acidic water mixes into the sand, some carbonates may start dissolving, sequestering CO2 in the process.

Laboratory-based sand columns (left) and field-based chamber (right) used to measure carbonate dissolution. In both experiments, acidic water replicating future ocean conditions is pumped through the sand.
I’m just starting to analyze my data, but it’s already clear that significant levels of carbonate dissolution will occur, even at near-future ocean conditions. This information will help predict the chemistry of California’s coastal water in the near-future. On a larger scale, it will also help ocean and climate modelers forecast global ocean/climate conditions. Extending the results of my research even further, we may be able to learn from the “natural” dissolution I’m measuring, and engineer new methods for carbon sequestration using acidic water and carbonate dissolution. Hence, although this research measures local processes, happening right under our feet (at low tide) or boat (at high tide), it could ultimately be important far beyond California!
Before signing off, I want to send a big thank you to everyone who has supported my work. I’m funded by the Victoria J. Bertics Fellowship, which supports PhD research on sediments, and carries on Dr. Bertics’ enthusiasm for benthic ecology and chemistry. I also thank the incredible Wrigley staff for their infinite patience and dedication! And thank you for reading!