Hey everyone! My name is Cara Schiksnis and I’m going into my third year in the Marine Biology and Biological Oceanography PhD program at USC. I’m a member of the Hutchins lab where I study climate change effects on cyanobacteria, including studying the physiological and molecular response of cyanobacteria to the possible interactive effects of warming and nutrient limitation. These two stressors will become increasingly prevalent in the future ocean; in fact, warming of the ocean’s surface layer will lead to a reduced input of nutrients into the euphotic zone through a process called stratification. So, studying warming and nutrient limitation simultaneously is a more realistic way to try to understand how phytoplankton will react when both occur at the same time.
Why should we care about cyanobacteria, or any phytoplankton for that matter, you may ask? Believe it or not, marine phytoplankton (plankton that photosynthesize) contribute to 50% of carbon fixation on the planet (take that, trees), and thus not only hoist up the rest of the marine ecosystem/ food web but contribute immensely to biogeochemical cycling in the ocean. Thanks to these small but mighty creatures, the ocean regulates climate by absorbing carbon dioxide and sequestering it away from the atmosphere. Understanding how climate change will affect primary producers is essential for better predicting how these important, largescale climate processes may also vary. I could go on, but long story short all of these tiny microbes don’t get nearly enough of the credit and love that they deserve and it’s important that we continue to seek to understand how they may change as anthropogenic climate change continues to worsen.
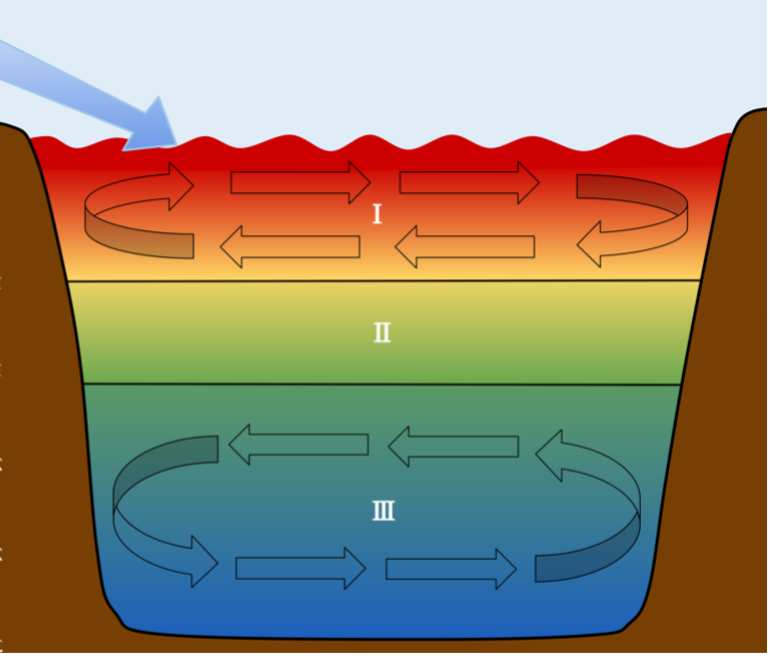
As the upper layer of the ocean warms, stratification increases while circulation, including input of new nutrients, decreases
With the Wrigley Fellowship, I was able to take my experiments out of my lab at USC, where I use unialgal (one species) cultures, to the lab at Wrigley, where the ocean access allowed me to collect natural seawater and its associated microbes in order to study these climate change effects on a community-wide level! Experimenting with natural communities can allow us to answer broader questions that relate to species interactions and food web dynamics. Plus, simulating climate change conditions on samples plucked straight from the ocean can oftentimes paint a more realistic picture of how these effects might actually play out. So, thanks to Juan, Dramamine, and my trusty bilge pump, I took my shaky sea legs for a spin and collected many liters of seawater, which I then took back to the lab and used to start to grow my experiments!

Bilge pumps are traditionally used to remove water from the boat, but here is me doing the exact opposite
Through the haze of the Dramamine, I first hit the filter rig to collect “T0” samples so I would have a baseline to which I could compare my experimental treatments later on. Then, I aliquoted seawater into culture bottles, spiked them with nutrients, and set them to incubate in temperature- and light- controlled incubators. I created 2 nutrient treatments (silica (Si)-limited and Si-replete) and 3 temperature treatments (20C, 25C, and 30C), with 3 replicates of each, for a total of 18 bottles. My *hope* was that I would observe differences in growth and community structure between these treatments, and that perhaps there would be an interactive effect between warming and Si limitation. Recent work has shown that warming may cause some cyanobacteria to cope better with iron limitation, possibly due to increased metabolic efficiency, but whether this effect plays out with Si within coastal communities has yet to be explored.

After splitting into bottles and creating nutrient treatments, I let the replicates grow at 3 temperatures.
I collected data such as cellular carbon, nitrogen, phosphorous and silica content, carbon fixation rates, photosynthetic pigment (Chl a) content, and DNA for assessing community structure. Results from an initial experiment I ran at the beginning of the summer interestingly show that the Si-limited communities grew the fastest at the warmest (30C) temperature, and even grew faster than the 30C, replete treatment. This indicates that warming might actually benefit nutrient limited communities; perhaps if the Si-limited community is dominated by smaller, non-siliceous cells such as cyanobacteria or smaller algae as opposed to larger diatoms, it is better equipped to thrive at higher temperatures. I am curious to continue analyzing these results and also to discover how the community structure changed as a result of these nutrient and temperature conditions.
As I wrap up my final days of experimentation out at Wrigley, I am able to reflect on and appreciate this unique and incredible research experience I’ve been lucky enough to receive this summer. A big thanks to all the Wrigley staff (especially with their help powering my experiment through the power outages), my support back at the Hutchins lab, and the oceans for making all of this possible- it’ll be a summer I won’t forget and I hope to be back on this dreamy island very soon!