By: Nick Orchanian
Hi everyone! My name is Nick Orchanian and I’m a PhD student in the Chemistry Department here at USC working with Professor Smaranda Marinescu. This summer I’ve been lucky enough to focus all my time on new and exciting research, thanks to the Norma and Jerol Sonosky Summer Fellowship, and I’m writing this post to hopefully get you excited about it as well!
I’ve always been interested in the ways we generate energy to power our world and build new products and materials, so my research now ties together these very different problems. Although fossil fuels have allowed us to achieve some amazing things as a society (building skyscrapers, powering hospitals, creating anything imaginable with the discovery of plastics, and many others), they can also trap us into depending on foreign powers for their oil or giving up our beautiful natural environments to dig up our own. To make an energy-independent America, we need new technologies to harness the greatest resource we already have here waiting for us: the sun!
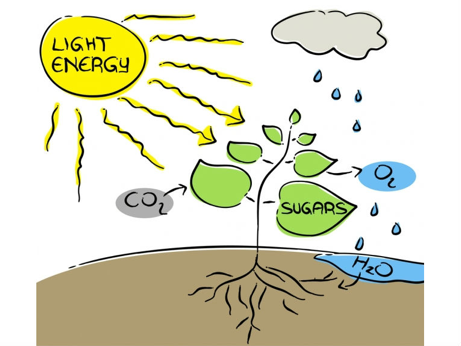
Photosynthesis turns carbon dioxide into food (sugars) and oxygen using solar energy. Image: https://www.shutterstock.com/image-illustration/photosynthesis-71587678
My research focuses on using sunlight to power chemical reactions on a big scale. There are all kinds of reactions you could try to power with sunlight, but my main interest is “artificial photosynthesis.” Photosynthesis is a series of chemical reactions that plants use to turn sunlight into sugar, which they (and we!) use to live and grow. Plants are able to do this using special “catalysts,” chemicals that act like little factories to change one kind of molecule into another (carbon dioxide gas into sugar). What we’d like to do is make our own catalysts that can convert CO2 into a huge range of important chemicals, including synthetic petroleum. By making this work, we could ultimately recycle the CO2 waste that’s generated by burning fossil fuels back into usable fuel.
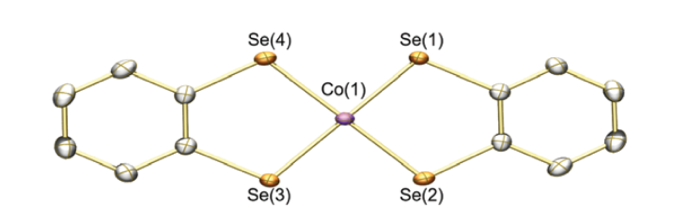
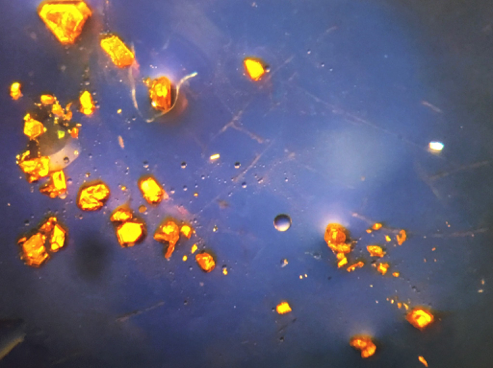
This is what one of our catalysts looks like: a small, flat molecule It has one cobalt atom (pink) sitting between two organic molecules made of selenium (yellow), carbon (gray), and hydrogen atoms (left out for clarity). By switching out one type of atom for another, you can change the way the molecule behaves. Image from our paper “H2 Evolution by a Cobalt Selenolate Electrocatalyst and Related Mechanistic Studies”
But making new catalysts is hard work. Sometimes a synthesis can take several hours or even days to finish, and growing nice crystals of your new molecule can take weeks! And the catalyst doesn’t always behave the way you’d like it to, so you need to take the time to rethink your design, sketch out a drawing for a new molecule with a different shape or size, bounce ideas off your coworkers, and then jump back in the lab to try again. This process opens up a lot of room for creativity: there’s 118 elements on the periodic table and infinite new ways to arrange them into brand-new molecules and materials. So even if your first try fails, there’s plenty more to discover.
The nice thing about our catalysts is that we use metals like cobalt, iron, and nickel mixed with small molecules (called “ligands”), and structures like these usually have bright, pleasant colors and grow into really cool crystals – like the ones I made above. We also get to use giant lasers, powerful magnets, X-ray guns, and extreme microscopes to study the new molecules we make.
Thanks for reading, and check out our other projects at http://marinescu.usc.edu/ if you want to learn more!
Nick