By Betsy Melenbrink
Hi folks! My name is Betsy and I am a 2016 WIES Sonosky Fellow starting the fourth year of my chemistry Ph.D. program at the University of Southern California. My graduate research is on solar energy, specifically polymer solar cells.
What is a polymer solar cell? Well, solar panels are increasing in popularity, and each panel is made up of many cells. A lot of schools, businesses, and houses now have shiny grey panels on their roofs. These solar panels are primarily made from silicon, an inorganic semiconductor, which is great at moving electrical charges through itself, but not very good at absorbing sunlight. This means that the panels must be manufactured to be very thick to absorb more light, a process which is very expensive and energy-intensive, and results in heavy, brittle panels. Alternatively, we can make organic semiconductors from primarily carbon-based molecules. Polymers are just long chains of these molecules strung together. The semiconducting polymers are great at absorbing sunlight, which means they can be incorporated into lightweight, flexible solar panels, and they can be manufactured very cheaply.
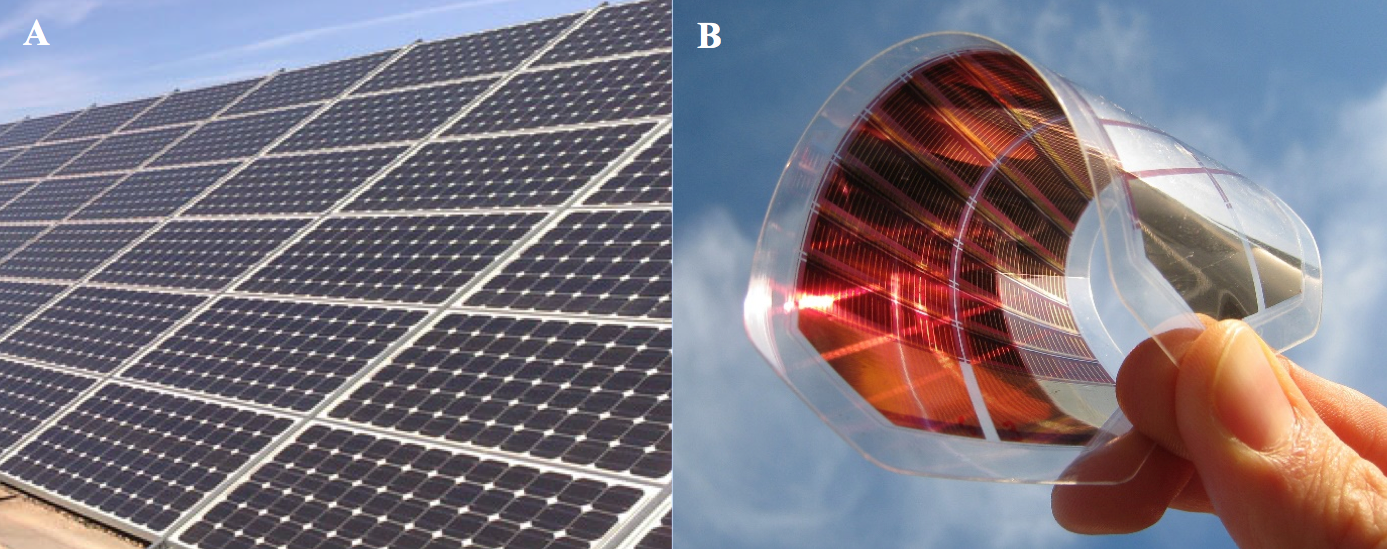
A) Silicon solar panels (figure taken from http://www.cnbmsolar.com/image/com-pic/company.jpg). B) Flexible organic solar device (figure taken from https://3dprint.com/wp-content/uploads/2014/03/solar-2.jpeg).
However, polymer solar cells currently have a few pitfalls as well. They are not yet as efficient at converting sunlight into electricity as silicon, and they do not have as long of a working lifetime. This is due to the fact that organic molecules can break down under intense sunlight and they can also move around in the thin film as it is heated by the sun. Both of these processes mean that the solar panel will lose its ability to convert sunlight into electricity as it ages.
My research aims to change the chemistry of the semiconducting polymers to make them more stable so that polymer solar cells will last longer. On a typical day, I make and purify monomers, a name that we use for the individual molecules that I later connect into long chains as polymers. I use typical organic chemistry methods for purification, such as extraction, column chromatography, and recrystallization.

A) An extraction to isolate a monomer. B) Shiny crystals obtained from a recrystallization. C) Separating out monomers using column chromatography
When my monomers are pure, I connect them together into long chain polymers. Even if my monomers are colorless, the resulting polymer is often very colorful, as in Image A below. After the polymers have been purified I subject them to a number of tests to determine their chemical structure, their molecular weight (how long the polymer chain is), how the polymers crystallize, and their electrochemical behavior (how much energy it takes to add or subtract an electron). Following all of this characterization, I can test the polymer performance in solar cells.

A) Leftover vials showing remains of colorful polymers. B) Purifying a colorful polymer using a soxhlet extractor. C) Betsy aligning a solar cell below the solar simulator – a lamp that imitates sunlight.
I am hoping to create solar devices that not only work immediately after they are made (as we usually test them), but also days, weeks, months, or even years afterward. By extending the working lifetime of these solar cells, I can help make the technology more viable as an alternative to silicon solar panels.
Betsy is a PhD student in the USC Department of Chemistry, in the research group of Dr. Barry Thompson. Her dissertation research focuses on development of lightweight flexible single-layer solar cells.
